Frontera's APEX Technology & Manufacturing Platform is an innovative adeno-associated virus (AAV) gene expression system. It includes novel and clinically validated AAV vectors, efficient and fully-integrated CMC processes, and access to early clinical data, making it possible to develop novel gene therapy products with quicker speed-to-market and lower cost.
- World-class execution – leveraging fully integrated teams – across disease biology, gene therapy vector design, translational sciences, process and analytical development, in-house GMP manufacturing and clinical research.
- State-of-the-art GMP facilities.
- World-class and proven team in gene therapy R&D, having been in leading roles or as founders for some of the most successful and innovative gene therapy and gene editing products and companies.
- Early access to clinical data made possible by Frontera’s unique scientific model.
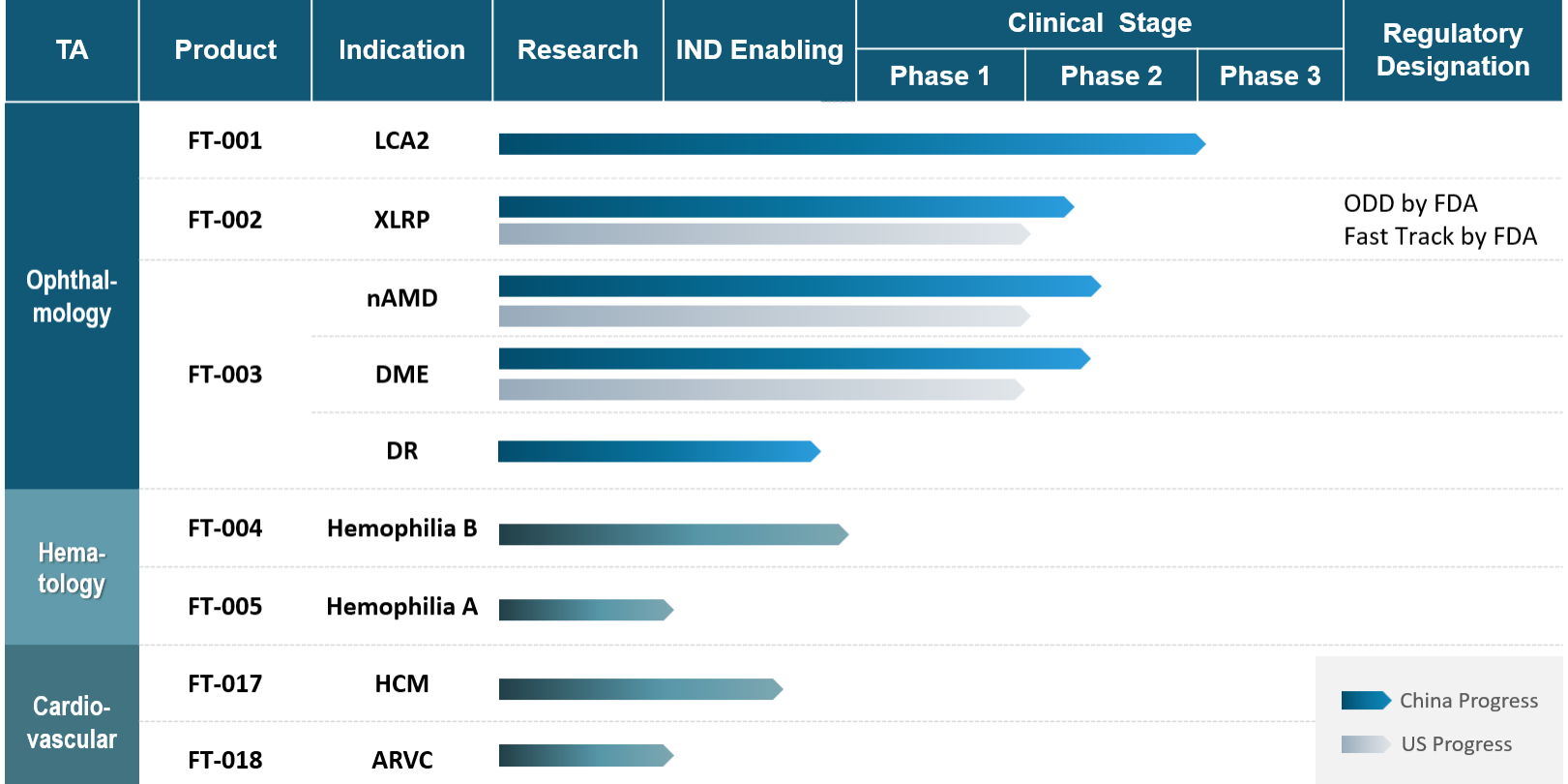
Pipeline
Product development in gene therapy has historically focused on a limited number of rare monogenic disorders. The possibilities for gene therapy, however, are vastly greater. Frontera is realizing this potential with a pipeline that spans across not only orphan diseases, but also in larger patient markets – including Ophthalmology, Hematology, Cardiovascular and Metabolic Diseases.
Board of Directors
Wei Li, Ph.D.
Chairman, Founding Partner of Creacion Ventures
Carl Gordon, Ph.D.
Managing Partner, OrbiMed Advisors
David Wang, M.D., Ph.D.
Partner, OrbiMed Advisors
Ching Zhu, Ph.D.
Founding Partner of Creacion Ventures
Xinyan Li, Ph.D.
Co-Founder & CEO
Yanling Cao
Partner, Boyu Capital
Jiang Han
Managing Director, Sequoia Capital China
Investors




Recent News
Frontera Therapeutics’ FT-002 Achieves Orphan Drug Designation from the U.S. FDA for Inherited Retinal Dystrophies
BEDFORD, Mass., and Suzhou, China, January 23, 2024 — Frontera Therapeutics announces that its pioneering AAV gene therapy product, FT-002, has received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of Inherited Retinal Dystrophies (IRD) caused by RPGR gene variants. About Orphan Drug Designation Orphan Drug Designation, granted […]
Read MorePreliminary Clinical Study Results of Frontera Therapeutics FT-002 Injection Presented at APVRS Congress
Hongkong, China, Decemeber 8th, 2023 – Preliminary clinical study results of FT-002, an AAV gene therapy treatment of Frontera Therapeutics, a potential first in class therapy for the treatment of XLRP, were reported at the 16th Asia-Pacific Vitreo-Retina Society, APVRS, 2023). Professor Sui Ruifang from Peking Union Medical College Hospital was invited to participate in […]
Read MoreFrontera Therapeutics Initiates Phase II Clinical Trial for FT-001 in Hereditary Retinopathy Treatment
Beijing, China, Decemeber 3rd, 2023 – Frontera Therapeutics (Suzhou) Co., Ltd. (hereinafter referred to as Frontera Therapeutics) convened a meeting in Beijing on December 3 to conclude the Phase I clinical study of FT-001, and initiate the Phase II clinical study. The meeting was attended by esteemed clinical experts, including Professor Sui Ruifang, Professor Yu […]
Read MoreFrontera Therapeutics announces CDE clearance to initiate clinical trial of a First-in-class gene therapy for the treatment of RPGR-associated X-linked Retinitis Pigmentosa
BEDFORD, Mass., and SHANGHAI, China, Nov 6th, 2023 – Frontera Therapeutics (Suzhou) Co., Ltd (referred to as Frontera) announced the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has given clearance of the Investigational New Drug (IND) application to begin a clinical trial of an innovative gene therapy drug, FT-002, for […]
Read MoreThe Frontera’s third gene therapy treatment has been approved by CDE for clinical studies
BEDFORD, Mass., and SHANGHAI, China, July 12, 2023 – An application for an investigational study in human patients with FT-004, an innovative gene therapy drug independently developed by Frontera Biotechnology (Suzhou) Co., Ltd. (hereinafter referred to as “Frontera Biotechnology”), was approved by the Center for Drug Evaluation (CDE) of China National Medical Products Administration for […]
Read MoreA first in China – Frontera Therapeutics Doses First Patient in a Clinical Trial of FT-003 Gene Therapy for the Treatment of DME
BEDFORD, Mass., and SHANGHAI, China, June 13th 2023 – Frontera Therapeutics announced another major clinical milestone: FT-003 was successfully dosed for the first patient with Diabetic Macular Edema (DME) in the Ophthalmic Hospital of Tianjin Medical University. This marks the first treatment of gene therapy for this indication in China. Read the original article Li […]
Read MoreFrontera Therapeutics Doses First Patient in a Trial of FT-002 Gene Therapy for the Treatment of X-Linked Retinitis Pigmentosa
Three of Frontera’s gene therapy product candidates have entered clinical trials since the beginning of 2023 BEDFORD, Mass., and SHANGHAI, China, February 16, 2023 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, announced that it […]
Read MoreFrontera Therapeutics Doses First Patient in a Clinical Trial of FT-003 Gene Therapy for the Treatment of Wet AMD
BEDFORD, Mass., and SHANGHAI, China, February 2, 2023 – Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, announced that it has dosed the first patient in a clinical trial of its innovative gene therapy product, FT-003, […]
Read MoreFrontera Therapeutics Doses First Patient in Phase 1 Clinical Trial for Gene Therapy FT-001 for the Treatment of Leber Congenital Amaurosis-2
BEDFORD, Mass., and SHANGHAI, China, January 6, 2023 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, today announced that it has dosed the first patient in a Phase 1 clinical trial of its lead gene […]
Read MoreFrontera Therapeutics Receives Additional IND Clearance for its Lead Program FT-001
China CDE accepts Investigational New Drug (IND) application for lead gene therapy product candidate, FT-001, for the treatment of a rare inherited eye disease. BEDFORD, Massachusetts, and SHANGHAI, China September 21, 2022 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of […]
Read More- « Previous
- 1
- 2
- 3
- Next »











