Frontera's APEX Technology & Manufacturing Platform is an innovative adeno-associated virus (AAV) gene expression system. It includes novel and clinically validated AAV vectors, efficient and fully-integrated CMC processes, and access to early clinical data, making it possible to develop novel gene therapy products with quicker speed-to-market and lower cost.
- World-class execution – leveraging fully integrated teams – across disease biology, gene therapy vector design, translational sciences, process and analytical development, in-house GMP manufacturing and clinical research.
- State-of-the-art GMP facilities.
- World-class and proven team in gene therapy R&D, having been in leading roles or as founders for some of the most successful and innovative gene therapy and gene editing products and companies.
- Early access to clinical data made possible by Frontera’s unique scientific model.
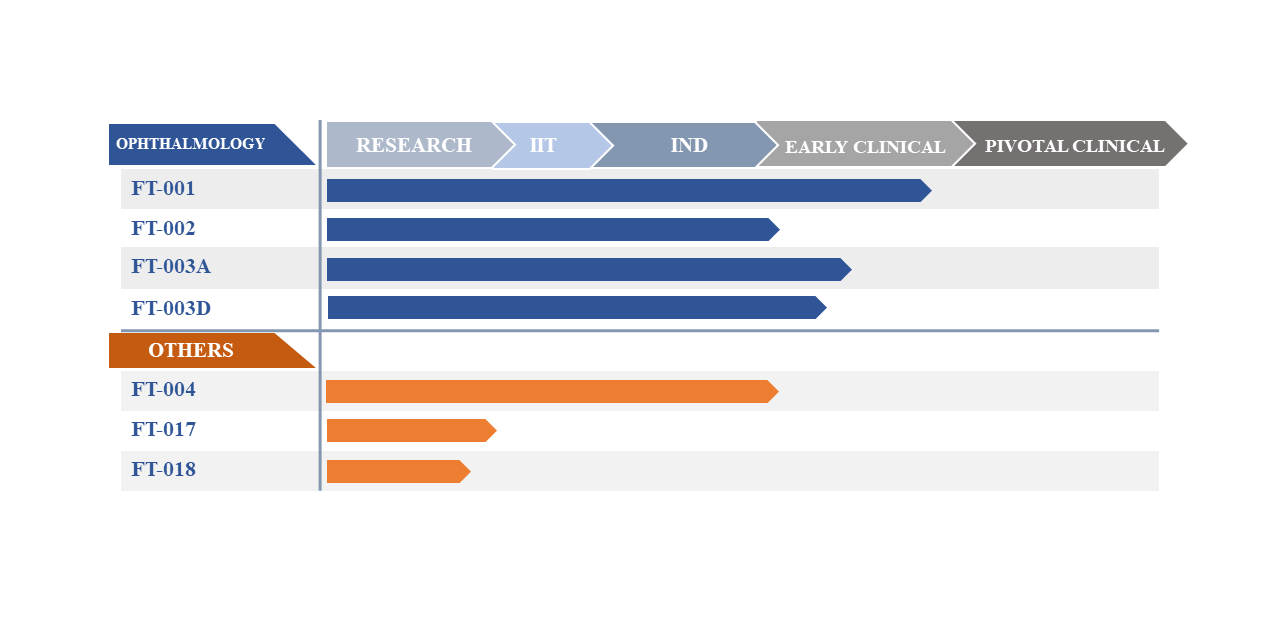
Pipeline
Product development in gene therapy has historically focused on a limited number of rare monogenic disorders. The possibilities for gene therapy, however, are vastly greater. Frontera is realizing this potential with a pipeline that spans across not only orphan diseases, but also in larger patient markets – including Ophthalmology, Hematology, Cardiovascular and Metabolic Diseases.
Board of Directors
Wei Li, Ph.D.
Chairman, Founding Partner of Creacion Ventures
Carl Gordon, Ph.D.
Managing Partner, OrbiMed Advisors
David Wang, M.D., Ph.D.
Partner, OrbiMed Advisors
Ching Zhu, Ph.D.
Founding Partner of Creacion Ventures
Xinyan Li, Ph.D.
Co-Founder & CEO
Yanling Cao
Partner, Boyu Capital
Jiang Han
Managing Director, Sequoia Capital China
Investors




Recent News
A first in China – Frontera Therapeutics Doses First Patient in a Clinical Trial of FT-003 Gene Therapy for the Treatment of DME
BEDFORD, Mass., and SHANGHAI, China, June 13th 2023 – Frontera Therapeutics announced another major clinical milestone: FT-003 was successfully dosed for the first patient with Diabetic Macular Edema (DME) in the Ophthalmic Hospital of Tianjin Medical University. This marks the first treatment of gene therapy for this indication in China. Read the original article Li […]
Read MoreFrontera Therapeutics Doses First Patient in a Trial of FT-002 Gene Therapy for the Treatment of X-Linked Retinitis Pigmentosa
Three of Frontera’s gene therapy product candidates have entered clinical trials since the beginning of 2023 BEDFORD, Mass., and SHANGHAI, China, February 16, 2023 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, announced that it […]
Read MoreFrontera Therapeutics Doses First Patient in a Clinical Trial of FT-003 Gene Therapy for the Treatment of Wet AMD
BEDFORD, Mass., and SHANGHAI, China, February 2, 2023 – Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, announced that it has dosed the first patient in a clinical trial of its innovative gene therapy product, FT-003, […]
Read MoreFrontera Therapeutics Doses First Patient in Phase 1 Clinical Trial for Gene Therapy FT-001 for the Treatment of Leber Congenital Amaurosis-2
BEDFORD, Mass., and SHANGHAI, China, January 6, 2023 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of patients across multiple disease areas, today announced that it has dosed the first patient in a Phase 1 clinical trial of its lead gene […]
Read MoreFrontera Therapeutics Receives Additional IND Clearance for its Lead Program FT-001
China CDE accepts Investigational New Drug (IND) application for lead gene therapy product candidate, FT-001, for the treatment of a rare inherited eye disease. BEDFORD, Massachusetts, and SHANGHAI, China September 21, 2022 — Frontera Therapeutics, a global clinical-stage biotechnology company that seeks to develop novel and best-in-class gene therapy medicines to improve the lives of […]
Read MoreFrontera Therapeutics Completes $160 Million Series B Financing to Fund Clinical Development and Manufacturing Capabilities
FDA clears Investigational New Drug (IND) application for lead gene therapy product candidate, FT-001, for the treatment of a rare inherited eye disease leading to severe visual impairment that may result in blindness. Investors in the $160 million Series B round include Boyu Capital, Sequoia China, and existing investors OrbiMed and Creacion Ventures, who were […]
Read More- « Previous
- 1
- 2











