-
-
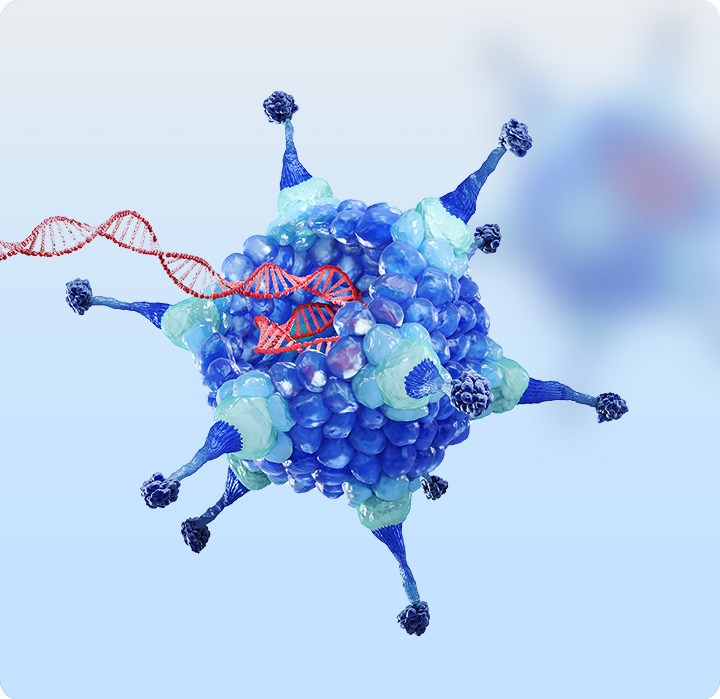
About rAAV
-
 Mechanism of Action
Recombination adeno-associated virus (rAAV) vectors carry a target gene of interest (GOI) to transduce specific tissues and cells. This enables continuous expression of functional proteins, repair of damaged cells, restoration of tissue function, improvement of clinical symptoms, and reversal of disease progression.
Mechanism of Action
Recombination adeno-associated virus (rAAV) vectors carry a target gene of interest (GOI) to transduce specific tissues and cells. This enables continuous expression of functional proteins, repair of damaged cells, restoration of tissue function, improvement of clinical symptoms, and reversal of disease progression. -
 Clinical Value
For hereditary diseases where no effective treatments currently exist, AAV gene therapies provide promising approaches with the potential to achieve lasting cures
Clinical Value
For hereditary diseases where no effective treatments currently exist, AAV gene therapies provide promising approaches with the potential to achieve lasting cures
For chronic diseases requiring lifelong medication, AAV vectors provide a "single-dose, long-term benefit" regimen. This significantly reduces medical burdens on patients while offering strong pharmacoeconomic advantages.
-
R&D Technology Platform (EXACTE™)
Frontera's R&D platform, EXACTE™ (EXcellence in AAV Capsid and Transgene Engineering), is a cutting-edge integrated system to enhance the accuracy, efficiency, and safety profile of therapeutic gene delivery. EXACTE™ is designed to accelerate the development of gene therapies through precision engineering and targeted delivery. Supported by an integrated in vitro and in vivo screening system, EXACTE™ enables the selection of drug candidates with optimal tissue tropism, high transduction efficiency, durable expression, and a strong safety profile, driving superior clinical outcomes and competitive cost structure.
-
Capsid Engineering
Engineer and screen optimized capsids for efficient delivery of therapeutic transgenes to target tissues, including the eye, liver, and heart.
Design proprietary RepCap constructs tailored for the baculovirus/Sf9 production system, enabling the generation of highly potent, full AAV particles.
-
Payload Engineering
Optimize codon sequences, including codon adaptation index, cryptic splice sites, CpG motifs, repetitive elements, and unfavorable RNA structures, to improve transgene expression.
Refine regulatory elements, including enhancers, promoters, introns, Kozak sequences, and poly(A) signals, to enhance transcriptional and translational efficiency.
Engineer payload constructs to improve vector yield, purity, and consistency during manufacturing.
-
Candidate Screening
Develop comprehensive potency, activity, and functional assays to evaluate capsid performance and transgene expression.
Establish and select appropriate in vitro and in vivo models for robust efficacy screening.
Conduct multi-model toxicity evaluations to assess safety and de-risk lead candidate selection.
Production Technology Platform (AAVANCE™)
Frontera operates a state-of-the-art GMP production facility in Suzhou China. Our AAVANCE™ (AAV Advanced Next-gen CGMP Excellence) manufacturing platform ensures comprehensive quality control over product supply and supports all stages of drug development (preclinical, clinical, and commercial stages). Our superior manufacturing capabilities not only accelerate the product development timeline but also significantly improve AAV yield and purity, enabling efficient delivery of high-quality gene therapies for patients.
-

Industry Leading AAV Production Platform
Large Scale: Utilizing the Sf9 insect cell expression system, we have established GMP production capacities of 200L and 500L.
High Yield: bioreactor yields consistently exceed 1E15 vg/L, reflecting an industry-leading level.
High Potency: Our vector designs, optimized for Sf9 cells, yield highly active AAV viruses.
Low Cost: Streamlined processes significantly reduce direct production costs, improving affordability for patients.
-

Innovative Purification Process
We maintain empty capsid levels below 1%, greatly enhancing product safety and creating a strong competitive advantage.
Downstream purification yields reach 50%, setting another industry benchmark.
-

Comprehensive Quality System
We have established a full-spectrum AAV quality control and assurance system, ensuring robust and consistent product quality.
Our platform meets regulatory standards across the United States, European Union, and China for gene therapy products.


 沪公网安备31011502401398号
沪公网安备31011502401398号